We study how taste cells function
A taste bud from a transgenic mouse that expresses GFP in taste receptor cells that express the taste specific G protein, gustducin. These cells are separate from taste cells that express the presynaptic protein, SNAP-25 (red labeling). There are several distinct cell types in a taste bud.
Taste receptor cells can be classified by their sensitivity to different types of taste stimuli and differences in the signaling pathways they use. Type II cells detect bitter, sweet or umami stimuli while Type III cells detect salt and sour. We identified a novel taste cell type that detects multiple types of taste stimuli and are broadly responsive (BR) to the chemicals in food. Artwork courtesy of Jhanna Flora
Taste cell signaling pathways
The taste system is extremely heterogeneous and is made up of different cell types that depend on multiple signaling pathways to detect stimuli. Some stimuli interact with receptors that initiate second messenger cascades, while others interact directly with ion channels to cause a cellular response. We recently identified a new signaling pathway in taste cells that contributes to the detection of certain taste stimuli. As characterization of these cellular mechanisms continues, we can begin understanding how the brain gathers information about its surroundings. One major goal of the lab is to better understand how different signaling mechanisms contribute to the detection and perception of taste stimuli. Current studies using calcium and sodium imaging along with molecular techniques are focused on defining the role of each of these pathways.
Defining the role of broadly responsive taste cells
We identified a previously unknown population of taste cells that are important for the transmission of taste information to the brain. These taste cells are broadly tuned and can respond to multiple types of taste stimuli, unlike the established taste cell types that detect a single stimulus type. These broadly responsive taste cells are required for the normal transmission of taste information but their contribution is not yet well defined. Ongoing studies in the lab are focused on understanding how these taste cells function.
Broadly responsive taste cells use a PLCb3 signaling pathway that is found in a subpopulation of Type III taste cells. Green-SNAP25, Magenta-PLCb3
TRPM4 (red labeling) and TRPM5 (green, GFP) are highly co-expressed in peripheral taste cells. TRPM5 is exclusively expressed in Type II cells but TRPM4 is present in multiple taste cell types.
Broadly responsive taste cells are a subset of Type III taste cells (identified by their responses to 50mM KCl (HiK)) that detect multiple types of taste stimuli, including bitter, sweet, umami and sour. This is in contrast to other taste cell types that have selective response profiles. Left-Taste evoked responses in BR cells, Right-summary of response profiles in Type III cells. BR cells are a subpopulation of the Type III cells.
Dutta Banik et al. (2020)
Defining the role of TRPM4 in taste signaling
We identified a pivotal role for the TRPM4 channel in the signaling pathways of Type II taste cells that detect either bitter, sweet or umami stimuli. TRPM4 and TRPM5 are monovalent selective channels that produce sodium signals in response to bitter, sweet and umami stimuli. These channels work in conjunction to generate an appropriate cellular response to taste stimuli. When either channel is absent, the ability of taste cells to respond to these stimuli is impaired. Ongoing studies in the lab are focused on understanding how these two channels work together within taste signaling pathways to produce an appropriate output signal.
We developed a dual-imaging approach to measure the cytosolic calcium (black line) and sodium responses (red line) to different taste stimuli. We found that many taste stimuli activate increases in both cytosolic calcium and sodium and that the sodium responses depend on both TRPM4 and TRPM5. When these channels are absent, the evoked cytosolic sodium response is lost.
Dutta Banik et al. (2018)
Diet-induced obesity and taste cell signaling
The taste system also has important, though poorly characterized, roles in appetite and food intake. When the relationship between taste and appetite is damaged, eating problems such as malnutrition and obesity can occur. The lab is currently interested in deciphering the relationship between obesity and taste. We have found that diet induced obesity has significant effects on the properties of taste receptor cells and current studies are focused on understanding how this happens.
The taste evoked calcium signals in taste receptor cells are significantly impaired in mice that were fed a high fat diet and became obese.
There is selectivity in how diet induced obesity affects taste. For some stimuli, increased weight (and the bodily changes associated with that) is the only factor that causes inhibition of taste responses (see summary of stimulus signal sizes, upper left, and taste driven behaviors, upper right, for AceK). For other stimuli (saccharin, lower panels) exposure to the high fat diet alone was sufficient to cause deficits in the stimulus responses and behavior.
Taste cell signaling in pigs
We have recently started characterizing the peripheral taste system in pigs. Pigs are important livestock animals and are increasingly used as a model system for human health. Currently, we don’t know much about their taste system. Our preliminary studies have identified that pigs express at least some of the same markers found in other mammalian taste systems. This provides a good starting point to study the cellular functions important in pig taste. A better understanding of the taste system in pigs will provide insights into relationship between taste and consumption in pigs which is important for their growth and overall health. This project is also predicted to validate the use of pigs as a better animal model to study taste and consumption in humans.
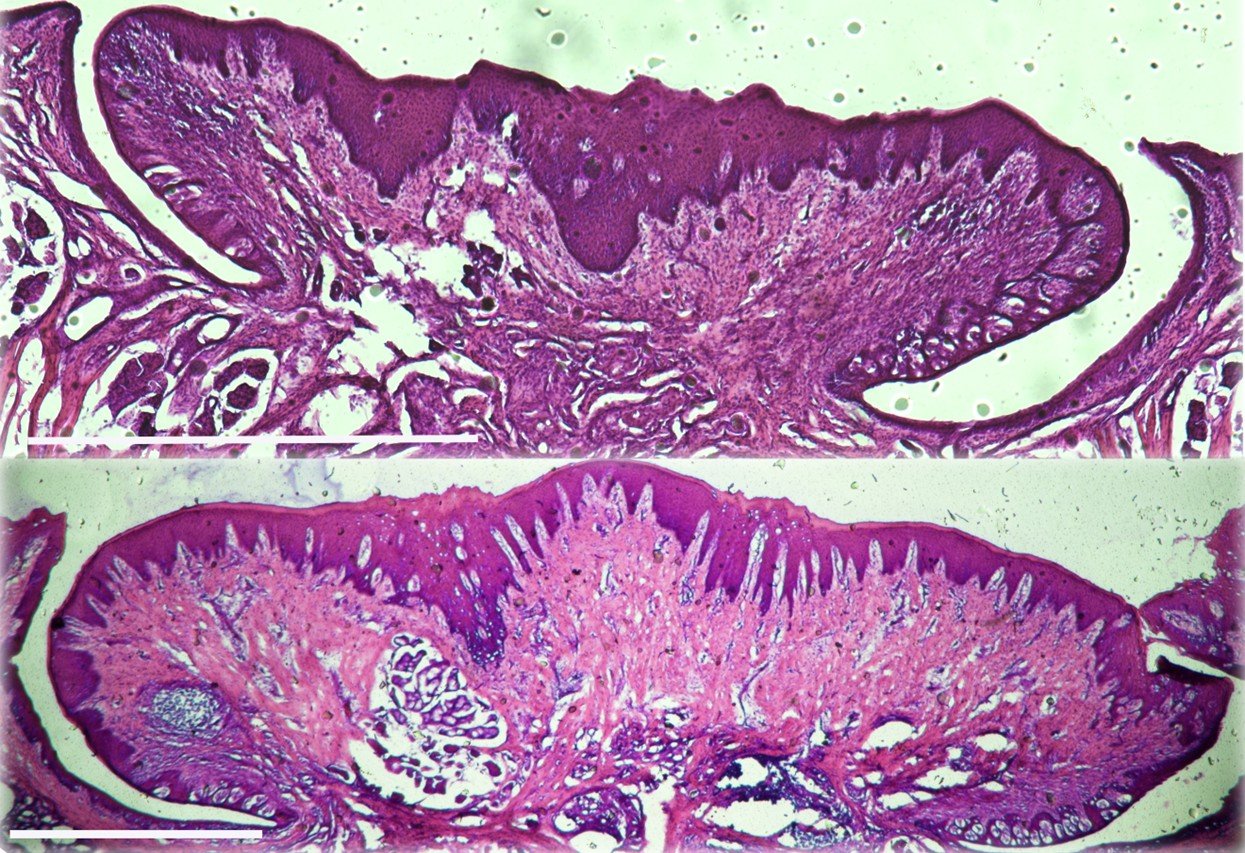
Taste papillae increase in size with age in pigs. Coronal sections of the circumvallate (CV) papillae from a juvenile (3wk, top panel) and an adult (5mo, lower panel) pig were collected and subjected to H&E staining. Representative sections are shown. Scale bar =1 mm for each.
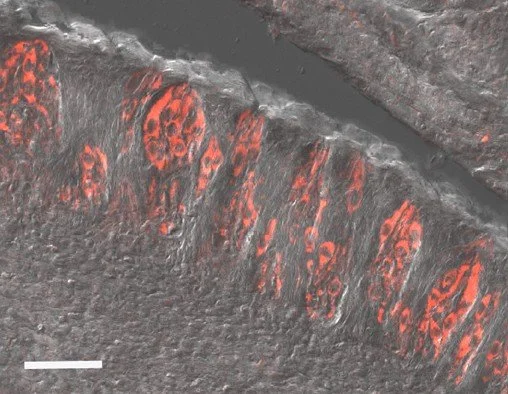
Pig TRCs express known taste signaling proteins. Immunohistochemical analysis of the taste buds in an adult pig CV papillae identified PLCbeta2 (Type II marker) expression. Scale bar = 50um.
Transcriptional regulation of taste cell maintenance
Taste cells are unique in that they have characteristics of both neurons and epithelial cells. Like neurons, they are excitable cells, form synapses and fire action potentials but like epithelial cells, they are routinely replaced throughout an organism’s life. Cell turnover and maintenance is not well understood and many factors likely contribute to this process. We found that the transcription factor, WT1, which is important in cell growth and differentiation, is expressed in taste cells. Our initial studies have found that WT1 is required for the normal development of the peripheral taste system. In adult taste cells, WT1 activity is suppressed by the transcriptional regulator BASP1 and data suggests this suppression of WT1 activity is needed to maintain cell differentiation. Current studies in the lab are focused on elucidating the role of the BASP1/WT1 complex in maintaining the differentiation of the peripheral taste system.
The WT1-BASP1 complex represses LEF1 and PTCH1 expression in the differentiated taste cells from mice.
(Left panels) Quantitative PCR (qPCR) was used to detect LEF1 (above) and PTCH1 (below) expression relative to GAPDH in mRNA isolated from taste cells of CTL and BASP1-KO mice. When BASP1 is absent, RNA levels of these two genes significantly increase. Since these genes are important for the initial development of differentiated taste cells, this suggests that normal differentiation features of taste cells are lost. (Right panels) ChIP analysis of isolated taste cells from adult mice. WT1, BASP1 or control (IgG) antibodies were used. After normalization to input DNA, fold enrichment at the LEF1 (top) or PTCH1 (bottom) promoter regions over a control genomic region is shown in CTL and BASP1-KO mice. These data demonstrate that WT1 is still bound to the gene promotors, even when BASP1 is absent. (Bottom panels) Immunohistochemical analysis of LEF1 expression in the circumvallate papillae (CV) that house hundreds of taste buds from either CTL or BASP1-KO mice (left panels). The expression levels of PTCH1 in the CV papillae of CTL or KO mice is shown in the right panels. Both proteins are upregulated when BASP1 is absent.
Taken together, these data suggest that when BASP1 is absent, taste cells lose their differentiation features due to the upregulation of the WT1 target genes that are important for the initial cell differentiation but are then suppressed to maintain the taste cells in their differentiated state.
Gao et al., 2019